ViiV Healthcare Company Overview
• ViiV Healthcare is an independent, global specialist HIV company founded in 2009 with a commitment to deliver innovative new options for the care and treatment of people living with HIV/AIDS.
• Joint venture between GSK, Pfizer, and Shionogi.
• Majority owned by GSK. Leverages GSK for operational support in many areas.
• More than 1,000 employees based in 15 countries and 3 regional hubs. Reported revenue of £4.7 billion (2018).
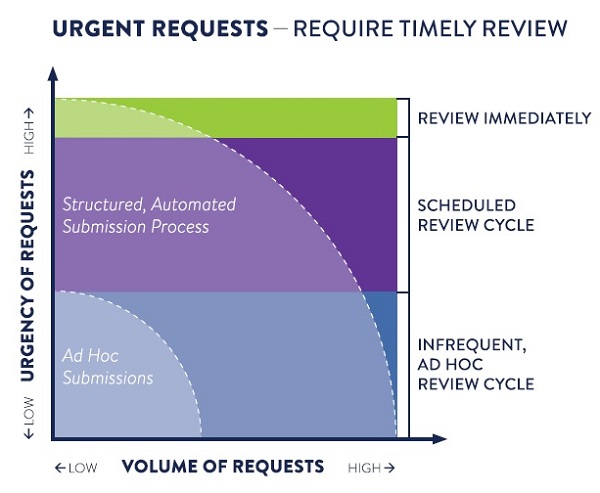
Problem: Urgent, Yet Ad Hoc Processes
Often, when an organization looks to automate or streamline a decision-making process, it’s because they want to handle a large volume of requests efficiently. But, what if the ability to scale is not the main driver? What if the primary concern is the urgency of making a decision and the consequences of taking too long?
Sometimes, it is a matter of life and death, and time-to-decision is critical. Health Care Providers (HCPs) need to know that their requests to access investigational medicine for compassionate use will quickly get to the right person at a pharmaceutical company, so they can determine if a drug may be a suitable fit for a patient in dire need.
Typical ad hoc processes for intake and decision-making don’t cut it for such urgent matters that require rigorous documentation, regulation, and multiple approval processes. Without an efficient system to streamline, manage, track and correctly route and complete requests, eligible patients might not receive the potentially life-saving medications they need in time.
It’s this problem that ViiV Healthcare wanted to solve within its Compassionate Use (CU) program.
ViiV’s goal is to leave no person living with HIV behind, which is why ViiV makes some investigational medicines available through compassionate use. This posed a set of unique challenges.
Since ViiV is an organization with global reach, the challenges ranged from manually managing the incoming requests, routing them to the appropriate internal specialist, coordinating with the requesting physician, ensuring the internal team had sufficient information to make a decision, conforming with country-specific regulations, and creating an internal process to make quick and medically sound decisions. Relying on spreadsheets and emails alone would be too cumbersome and too risky.
“ As the only company solely focused on HIV, we go beyond developing new medicines. We take a holistic approach to HIV by developing and supporting sustainable community programs — with and for the HIV community.” – ViiV Healthcare
The Solution: Centralized Request Process, Empowered Decision – Makers
ViiV Healthcare decided to implement ideaPointTM software from Anaqua to support a structured, semi-automated process for intake of compassionate use requests for HIV treatment. The company focused on improvements in two areas: centralizing request intake and streamlining the decision process.
Request Intake:
• HCPs can now request access to investigational medicines through a ViiV branded ideaPoint web portal. The portal includes information about which drugs may be requested, ViiV Healthcare’s compassionate use policy, and a secure mechanism by which an HCP can make a formal request and track the status of the compassionate use application.
• Once the request is submitted, the application is automatically routed by the ideaPoint software to the appropriate internal physician, who can then contact the HCP directly to obtain a clearer understanding of the patient’s specific circumstances and the reasons behind the compassionate use request. The HCP receives an immediate, automated response when the initial request is made; and a ViiV physician will reach out directly to the HCP within five business days or sooner, depending on the urgency of the request.
Decision-Making:
• Once ViiV has the essential information about the request, a small, cross-functional team meets to discuss whether the case fulfills pre-specified criteria for compassionate use. A decision to approve or decline the request is determined, and the decision is recorded within ideaPoint. Final decisions are ultimately signed off by ViiV’s Chief Medical Officer (which is also recorded in ideaPoint).
• If approved, ViiV procures and delivers the experimental agent.
• If declined, the ViiV physician either emails or discusses via phone the reasons for declining the compassionate use request.
• In either scenario, final interactions are documented in ideaPoint. ViiV’s goal is to make decisions in no more than 2-4 weeks of the request, sooner if there are extraordinary circumstances. ViiV also wants to track and log every step in the process to ensure compliance with their internal SOPs which is automatically done by the ideaPoint application.
The Results: Fast, Documented Decisions
By implementing ideaPoint software to inform HCPs, manage requests, and streamline and track all aspects relevant to the decision-making process, ViiV Healthcare has enhanced its internal process.
This allows for more expeditious evaluations and decisions regarding requests for compassionate use submitted to the company. ViiV is able to track all requests as they come in, make decisions in a timely manner, track the outcomes, and accelerate the process by which eligible patients can receive potentially life-saving medications prior to regulatory approval and wide-spread availability.
For more information about this use case, see the ideaPoint white paper: Best Practices for Streamlining Urgent Decision-Making within an Early Access Program




















