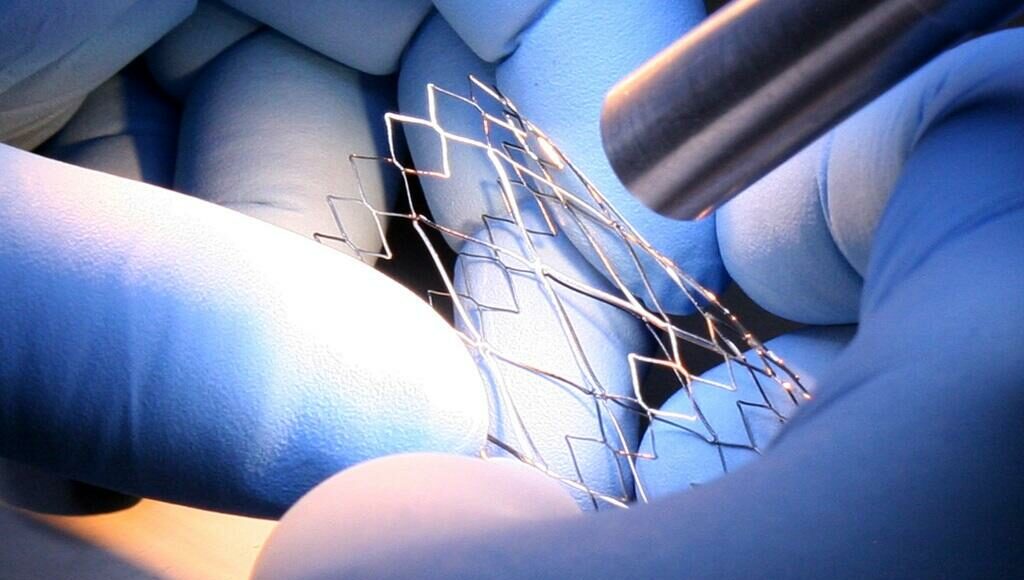
Concept-to-commercialization services include precision, tight-tolerance metalworking expertise and injection molding solutions; company also will display bio-based PVC compounds, antimicrobial compounding and reinforced paper for med device packaging.
Wayne, PA – TekniPlex Healthcare, which utilizes advanced materials science expertise to help deliver better patient outcomes, will highlight its broadened capabilities for designing and manufacturing Class III implantables and delivery systems at MD&M West, Booth 3201, February 4-6 in Anaheim. Now a full-fledged CDMO in these segments, the company’s capabilities span initial concepting, prototyping and pilot production through larger-scale manufacturing and commercialization. Other solutions TekniPlex Healthcare also showcase include bio-based PVC compounds, antimicrobial compounding, and its strongest-ever reinforced paper for medical device packaging.
Extended Capabilities for Medical Device Development
TekniPlex Healthcare is among the few medical device CDMOs offering comprehensive, ideation-to-completion product creation for endovascular stents, heart valves and delivery systems, among other categories. The company’s sweeping capabilities include high-precision, tight-tolerance development and prototyping for critical metal components typical in cardiovascular, pulmonary, gastrointestinal, laparoscopic and other endoscopic applications, as well as in chemotherapy administration.
TekniPlex Healthcare’s array of services supports customers from early ideation stages through product development, prototyping, clinical trials, testing, new product introduction and full production of catheter-based systems and other tubing-related solutions. This includes precision secondary processes and cleanroom assembly of Class II and Class III devices.
Bio-based Compounds for Medical Plastics Applications
Late last year, TekniPlex Healthcare became the first healthcare solutions company to introduce bio-based compounds for medical plastics applications whose performance and compositional properties are equivalent to conventional polyvinyl chloride (PVC) resins. Certified through the globally recognized ISCC PLUS system, the bio-based medical-grade PVC compounds utilize resins and plasticizers manufactured with renewable energy and bio-attributed classification. The compounds are equivalent to traditional medical-grade PVC in chemical composition, functionality and regulatory viability.
The bio-based compounds enhance sustainability profiles without sacrificing PVC’s reliable performance. For ease of manufacturability, the resins are drop-in replacements for a wide variety of injection molded or extruded PVC compounds, including those used for tubing solutions such as IV therapy tube sets, drip chambers, and peristaltic pump tubing; films used for trays, pouches, bags and other form-fill-seal settings; and other components composed of medical-grade plastics.
PVC and TPE Antimicrobial Compounds for Healthcare Applications
TekniPlex Healthcare also will emphasize its selection of antimicrobial medical compounds, which can be utilized to produce a number of patient-contact products including catheters, connectors, gas hoses, injection systems such as syringes, and needle-free injectors like pens. Available for formulation and production, the PVC and thermoplastic elastomer (TPE) compounds can incorporate silver ion technology proven to drastically reduce the risk of contamination due to bacteria buildup.
To develop its antimicrobial series of products, TekniPlex Healthcare integrates the silver ion additives – which have natural antimicrobial properties – with its broad array of current medical grade flexible and rigid PVC compounds, or its medical grade CELLENE® TPE compounds. Unlike disinfectants that last just a few hours, formulating a medical product with antimicrobial compounds provides protection throughout its lifespan.
Expanded Tyvek® Converting and Reinforced Paper for Med Device Packaging
At MD&M West, TekniPlex Healthcare also will showcase samples of its medical device packaging offerings, including a variety of grid lacquer-coated paper specifications and coated Tyvek® options. Notably, TekniPlex Healthcare recently expanded its converting capability for DuPont™ Tyvek® healthcare styles to include the EMEA region. For the company’s wide array of medical device customers in those geographies, the development will yield local sourcing and shorter lead times for market-leading coated solutions containing Tyvek® products.
TekniPlex Healthcare also will feature its strongest-ever reinforced paper for medical device packaging applications. The HPC74 Series features the highest puncture and tear strength of all TekniPlex Healthcare reinforced papers to date, as well as outstanding porosity levels. Available in various proprietary coating formulations, the high-performing, breathable medical-grade paper provides a clean peel and wide processing windows for rollstock and lidding applications.