In an interview with Nigel Theobald, CEO of N4 Pharma, several key points emerged regarding the company’s recent strategic moves and its outlook in the pharmaceutical landscape.
1. Can you provide more insight into the decision to acquire a controlling interest in Nanogenics Limited and how this partnership will enhance N4 Pharma’s product offerings?
Non-viral, non-lipid delivery systems are in high demand in the gene therapy space and though the acquisition of Nanogenics and the addition of LipTide®, N4 Pharma now has two such delivery systems and we expect to see considerable technical synergies in developing programmes using them both.
N4 Pharma is a pre-clinical stage specialist pharma company offering Nuvec®, a unique patented silica nanoparticle delivery system, for the reformulation and development of cancer treatments and vaccines. It is currently funded for proof-of-concept work investigating dual loaded small interfering ribonucleic acid (siRNA) nanoparticles to reduce cancer resistance and additional funding was raised for the acquisition opportunity with Nanogenics, to broaden participation in this sector.
LipTide ® is a complementary lipid and peptide-based delivery system which is being used to develop a novel siRNA product targeting an unmet clinical need in the ophthalmology market – ECP105. The RNA sector is an exciting one with a lot of investor and commercial interest so the addition of the LipTide ® delivery system and siRNA sequence adds significant potential value to our business.
2. Could you elaborate on the unique features and advantages of N4 Pharma’s Nuvec® delivery system for cancer treatments, gene therapy, and vaccines compared to traditional delivery methods?
Lipid nanoparticles (LNPs) present several challenges as a traditional delivery method including: particle size and limited formulation options, compatibility and safety, protection and targeting, uptake and efficacy, metabolism and clearance, as well as cost and scale-up potential. In response to these limitations, mesoporous silica nanoparticles (MSNs) have emerged as a promising alternative avenue for drug delivery. Characterized by their small size, inert properties, and expansive specific surfaces amenable to functionalization with therapeutic and targeting agents, MSNs offer a compelling solution.
In particular, Nuvec ® has been engineered with a unique spiky structure to efficiently and safely transport siRNAs, and other nucleic acids, to designated cells. As Nuvec ® protects the payload from enzymatic digestion and pH exposure, it holds potential as an approach in overcoming many of the challenges presented by LNPs. Other key benefits include thermostability; Nuvec ® can be dried, stored at room temperature and reconstituted without degradation, so there is no need for expensive cold chain storage and; ease of manufacture and chemical modification to allow cellular targeting.
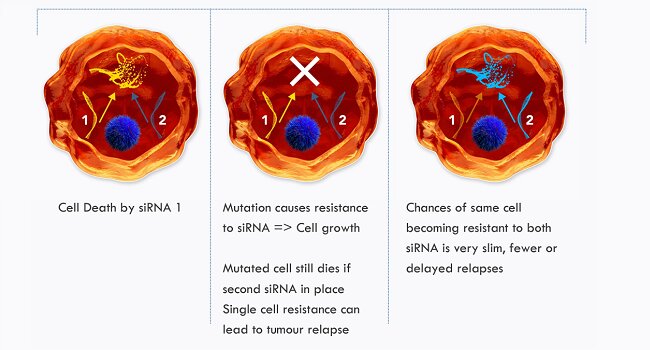
Nuvec ® also has the ability to deliver multiple siRNA to the same cell, by dual/multiple loading on one particle, which has the potential to help reduce tumor relapse during cancer treatments. Current delivery of dual siRNA requires multiple carriers, which decreases the probability of transfecting the same cell with more than one siRNA, thus hitting multiple aspects of a cellular pathway is less likely. Combination therapy is the main means of preventing resistance (e.g. antibodies or small molecules), as siRNAs could knock down two targets on one pathway, or two different pathways, making it a safer and more efficacious alternative to conventional chemotherapy.
3. How does N4 Pharma plan to navigate the regulatory approval process with regulatory bodies such as the FDA?
The Food and Drug Administration (FDA) has authority to grant orphan drug designation (ODD) to a drug or biological product to prevent, diagnose or treat a rare disease or condition. Being granted ODD can provide various benefits including market exclusivity, tax incentives, eligibility for grants, accelerated approval process and regulatory fee waivers. ODD aims to expedite the development of promising therapies by providing increased support and an accelerated regulatory framework.
N4 Pharma is exploring the option to get ODD for ECP105. We can prepare a compelling case, which we believe fits the ODD criteria, as we can demonstrate its potential to address an unmet medical need for non-viral delivery vehicles for ophthalmology and beyond for both RNA and deoxyribonucleic acid (DNA) payloads. We already have good efficacy data in vivo and if we get ODD, we can accelerate the regulatory process to get this beneficial treatment to patients in need as soon as possible.
4. Can you discuss the potential market impact of ECP105 using LipTide® for the treatment of glaucoma, including any projections for patient adoption and market penetration?
Glaucoma is a complex and multifaceted ocular disease characterized by progressive optic nerve damage and visual field loss. As a leading cause of irreversible blindness, it underscores the critical need for comprehensive understanding, early detection, and effective management strategies. In 2020, more than 75 million people worldwide were affected by glaucoma, highlighting its growing global prevalence. In 2021, the total glaucoma treatment market size exceeded 5.5 billion USD and is expected to witness over 3.2% CAGR between 2022 and 2028. With ageing populations, comes increasing rates of advanced or rapidly progressing glaucoma, which necessitates very low intraocular pressures, which can only be achieved through trabeculectomy.
There are treatments for glaucoma available that can help prevent the majority of patients from requiring this surgery, but for those that do, around 50% of glaucoma surgery results in fibrosis and rechallenge. ECP105 is designed to enhance glaucoma outcomes post-surgery, without the toxic side effects of the current alternatives – such as Mitomycin C – a chemotherapy drug which is used off-label in the US market.
In vitro proof of concept work demonstrated siRNA inhibition of proteins related to fibrosis and ophthalmic indications. There is also positive preliminary data for siRNA delivery and efficacy in vivo for prevention of glaucoma surgery failure.
Once approved for use in glaucoma treatment, there is broader market potential for this therapy in additional fibrotic indications including lung and liver.
5. Could you elaborate on the collaborative partnerships N4 Pharma has established, particularly with the University of Queensland, and how these partnerships contribute to the company’s research and development efforts?
Partnerships are a critical way for technology start-ups in this field to progress early-stage research, especially in an area like oral delivery. N4 Pharma has an ongoing partnership with the Australian Research Council at the University of Queensland, to investigate Nuvec®. Through in vivo testing it has already shown the successful oral delivery of a Nuvec® capsule into the intestine where it has released its plasmid DNA payload to produce localised and prolonged protein expression. This work demonstrates the potential of Nuvec® as an oral delivery system for multiple nucleotide payloads.
Further work with the University of Queensland is supported by a grant award to develop an improved nanoparticle delivery system that enhances the performance of messenger RNA (mRNA) therapies based on Nuvec®. N4 Pharma is working with Professor Chengzhong Yu to tackle the current challenges in manufacturing mRNA therapeutics by building new knowledge in custom-design of functional nanomaterials for mRNA delivery.
6. How does N4 Pharma plan to commercialize its products, both independently and through partnerships with other companies developing novel antigens?
N4 Pharma has recently entered into a research collaboration with SRI International Inc, a global research and development non-profit, based in Silicon Valley, California, to combine Nuvec® with SRI’s proprietary FOX Three Molecular Guidance System™. N4 Pharma will collaborate with SRI to perform research to conjugate its MGS system to Nuvec® in order to increase intracellular delivery to specific target cells and will co-market the combined technology.
SRI has demonstrated that the MGS technology can deliver more than a dozen types of payloads – from functional enzymes, antibodies, and nucleic acids including siRNA, antisense oligonucleotides (ASOs) and DNA – to targeted, intracellular molecular-target locations previously considered unreachable, and therefore, untreatable.
On the back of this combined technology, N4 Pharma will work closely with SRI to develop and pursue new business opportunities together building on SRI’s existing relationships with many of the companies developing novel biotherapeutics.
7. Can you provide insights into the potential scalability and manufacturing processes involved in producing Nuvec® and LipTide® for commercial distribution?
We have done quite a bit of work on scalability of the manufacturing of Nuvec® with pre-clinical volumes, having identified a partner CDMO, Ardena, who can produce Nuvec® to current good manufacturing practice (CGMP) standards. We have also identified the process of scalability that we need to go through with Ardena to get to the volumes required for clinical products.
For LipTide®, we are using microfluidics as a manufacturing methodology which recently came to the foreground with lipid nanoparticles for vaccine development. It requires a more established regulatory process and is very scalable.
8. Finally, what are the long-term goals and aspirations for N4 Pharma in the fields of oncology, gene therapy, and vaccines, and how does the company plan to position itself as a leader in these areas?
We know Nuvec® is innovative and potentially transformative. It works, it delivers, and it protects. Double siRNA and oral applications will make it extremely relevant to people working in this field. Our end goal is to get more effective therapies to more patients and we believe N4 Pharma has great potential to make strides in this area with Nuvec®, LipTide® and ECP105.
Our plan for Nuvec® is that, combined with the targeting technology from SRI, it becomes the go-to alternative to lipid nanoparticles for effective delivery of nucleic acids.
Nuvec® is available to license to partners for product development.






















