Randomized clinical trials have demonstrated that teduglutide, a glucagon-like peptide-2 (GLP-2) analog, improves intestinal absorption in patients with short bowel syndrome (SBS) with parenteral support (PS) dependence.1 Our objective was to assess real-world evidence of teduglutide effectiveness and safety in adult SBS patients with PS dependence in a real-world setting.
Methods
We searched PubMed databases for English language papers published through January 22, 2020. We completed a systematic literature review (SLR) to identify manuscripts (n=162) describing real-world evidence studies of teduglutide in adult SBS patients with PS dependence. Random effects meta-analysis of data identified in the SLR generated summary estimates, using the DerSimonian-Laird approach,2 for mean change in PS volume, mean change in PS infusion frequency, and percent of responders (≥20% PS volume reduction from baseline) by weeks 24 and 48 or end of follow-up (EOF). We also analyzed reported adverse event (AE) data. We present efficacy results as combined point estimates.
Results
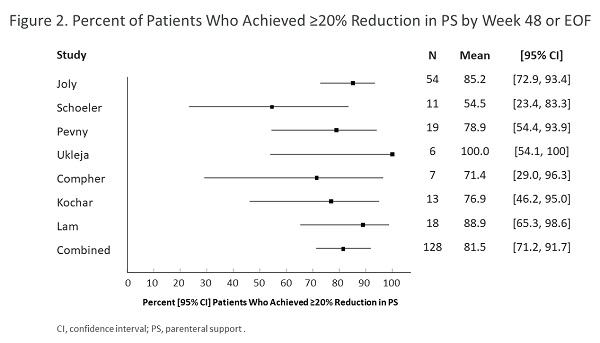
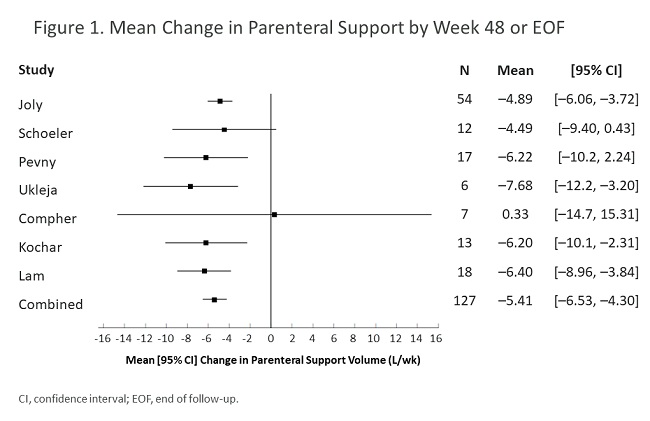
Seven studies involving 127 participants were included that investigated teduglutide. By week 24, PS volume changed by a combined point estimate (CPE) [95% CI] of –4.71 [–6.63, –2.79] L/wk and PS frequency changed by a CPE [95% CI] of –1.87 [–3.22, –0.52] days/wk. By week 48 or EOF, PS volume changed by a CPE [95% CI] of –5.41 [–6.53, –4.30] L/wk (Figure 1) and PS frequency changed by a CPE [95% CI] of –2.32 [–3.55, –1.09] days/wk. A CPE of approximately 70%-80% of patients responded (achieved ≥20% reduction in PS) to teduglutide by 24 or 48 weeks or EOF (week 48/EOF in Figure 2). AEs reported in 5 of 7 studies included abdominal pain (14.3%-50.0%), nausea/vomiting (7.7%-33.3%), catheter sepsis (16.7%-30.8%), and stoma enlargement in 50%-100% of patients with stomas. Four deaths were reported in 3 of 7 studies (none were associated with teduglutide per authors).
Conclusions
Although it is difficult to compare data from different settings, this meta-analysis of real-world data shows teduglutide efficacy was equal to or greater than that in randomized clinical trials for adults with SBS with PS dependence. Safety data appear comparable. Further research is needed on modifiable factors associated with best patient outcomes with teduglutide.
References
1. Jeppesen PB, Pertkiewicz M, Messing B, et al. Gastroenterology. 2012;143(6):1473-1481.e3.
2. DerSimonian R, Laird N. Control Clin Trials. 1986;7(3):177-188.

















![Sirio Launches Global Research Institute for Longevity Studies [SIA]](https://www.worldpharmatoday.com/wp-content/uploads/2019/09/Sirio-218x150.jpg)