By Daniel Canton, Dr. Daniel Haines, Dr. Uwe Rothhaar, SCHOTT pharma services
As the demands that are being placed on the quality and stability of medications continue to increase, the interactions that take place between the primary packaging container and filled drug product are becoming increasingly important. Even primary packaging that has been manufactured and stored properly can release substances into the drug formulation. For this reason, pharmaceutical manufacturers are required to conduct extensive studies on “Extractables” and “Leachables” (E&L).
Packaging systems often consist of many different components and materials, like glass, metal, plastic, rubber, adhesives or lubricants. Extractables are determined by subjecting the packaging material to aggressive conditions such as different solvents at elevated temperature for extended times, resulting in substances that elutefrom these packaging systems.
By contrast, Leachables are substances that migrate into a pharmaceutical formulation under normal preparation and storage conditions.Leachablesareusually, but not always,asubgroupofExtractables:for instance,asubstancecontained in the drug product formulation can react with a constituent contained in the packaging and form an entirely new species that is later identified in a leachable study, but would not be present in an extractable study.
The primary sources of”organic” Extractables and Leachables areelastomeric and polymeric materials likerubber and plastics,because they contain additivesthat allow for them to have beneficial properties, such as greater chemical stability and increased manufacturing yield. Here, it is important to know the exact composition of the packaging material in order to be able to perform E&L studiesefficiently. Nevertheless, this type of information often cannot be obtained from the material suppliers due to the complexity of the manufacturing processes,desire to protect their own process know-how, and potential upstream changes in raw materials. To address this issue, most testing laboratories that perform E&L studieshave built up comprehensive databases on materials and additives.
Most people are less aware of the fact that the original composition of the packaging system alone is not mainly responsible for what substances are found as E&L.One additional influencing factor is the sterilization process. As is well known, gamma irradiation can lead to polymer and additive degradation (e.g. crosslinking and scission), whereas steam sterilization can alter the mechanical and chemical properties as well (e.g. softening).
Regulatory requirements
Pharmaceutical companies need to conduct leachable studies to prove that no harmful substances will penetrate from the primary packaging into the drug during appropriate use of the drug in the respective dosage under normal storage conditions.This procedure is specified in a number of guidelines or regulations for the United States / Canada andfor Europe.They do not include any specific instructions on how to perform E&L studies, however.
It is important for pharmaceutical manufacturers to be able to come up with this proof as cost effectively as possible. Extractable studies help them to achieve this goal because they enable the toxicological assessment of possible Leachables and identity the number of substances that need to be tested with validated methods in the subsequent leachable studies. One of the challenges with E&L studies is that the study design has to be adapted to the drug composition and processing as well as the packaging properties.
Some recommendations
A working group at the Product Quality Research Institute (PQRI) based in Arlington, Virginia, has developed detailed recommendations on a special group of pharmaceutical products in which the risk of interactions with the packaging is considered to be particularly high. Some examples are published with respect to the dosage form: Thisincludesnasal sprays, oral inhalation aerosols and parenteral liquids, as well as best practices for representative parenteral and ophthalmic packaging materials.
The following aspects of extractable studies are particularly important:
1. Extraction should take place using a plurality of solvents of different polarities and using several different types of extraction methods. Prior workingknowledge of pharmaceutical formulations and packaging materials can help to minimize these efforts and choose the right solvents and methods.
2. Several different analytical techniques should be employed. Common suitable methods are: HS-GC/MS (headspace-gas chromatography/mass spectrometry) for volatile Extractables, GC/MS for semi-volatile, LC/MS (liquid chromatography/mass spectrometry) for non-volatile or polar Extractables,andICP-MS (inductively coupled plasma/mass spectrometry) for analyzingelemental impurities, e.g. metals fromorganic pigments or polymerization catalysts. With the increasing regulatory requirements, the analytical capabilities need to be improved and updated in short cycles especially with respect to lower detection and quantification limits.
3. Extensive experience, appropriate databases and reference materials,high qualified laboratory staff and proper quality control helps to interpret the data correctly to draw the proper conclusions and avoid mistakes.
Study design on the impact of sterilization
For example, the experts from SCHOTT Pharmaceutical Systems have observed that the extraction profile varies depending on the sterilization method used.“For some packaging components such as rubber stoppers, sterilization is mandatory before they can be used for medical purposes. However, Gamma irradiation can lead to polymer and additive degradation, whereas steam sterilization can also alter the mechanical and chemical properties. Both have an impact on the extraction profile,” says Daniel Canton, laboratory manager at SCHOTT pharma services.
To demonstrate this impact, his team has conducted a study with commercially available stoppers made of bromobutyl rubber for use in syringes. It included five different stoppers from three different manufacturers and three variants of sterilization: Steam sterilization (autoclaving, 121 °C for 30 minutes), gamma sterilization (40 kGy), and a combination of the two techniques gamma-irradiation (first) and steam-sterilization (second).
General Observation
The study shows that the total content of organic components (VOC, SVOC, and NVOC) deviates quite significantly between various bromobutyl stoppers from different manufacturers.In most cases,bromomethane and acetonecan be found prior to any sterilization. Their concentration significantly increases upon gamma-sterilization.
The total amount of SVOC strongly depends on the applied extraction solvent and method. Concerning extraction of rubber oligomers(side products of rubber formation), rubber degradation products and antioxidants extraction in dichloromethane is most efficient. In most cases, extraction of fatty acids – e.g. from palmitic or stearic acid derivates – seems to be more efficient using isopropanol -probably depending on kind of derivate contained in rubber formulation.
A steric hindered phenolic antioxidant can be found in all stoppers. Extraction of these antioxidants was most pronounced using dichloromethane as extraction solvent.
Impact of sterilization
The sum of VOC out of all samples significantly increases upon gamma-sterilization.Significant amounts of isobutene and other unsaturated hydrocarbons out of all stoppers can be observed only after gamma-sterilization.Generally, the sum of VOC decreases upon steam-sterilization of unsterilized and gamma-sterilized stoppers. That effect is also observed for single substances like isobutene, bromomethane, and acetone.
The concentration of extractable antioxidants out of all samples significantly decreases upon gamma-sterilization. This might influence the stability of the base polymer material and lead to formation of radical degradation or pyrolysis products of the basic polymer, e.g. formation of isobutene or bromomethane. Steam-sterilization had no significant influence on antioxidant concentration.Extractable heptadecane- as well as some other saturated and unsaturated hydrocarbons – out of all samples can be found only upon gamma-sterilization and gamma- with additional steam-sterilization.
In general, the total amount of extractable palmitic and stearic acid out of all stopper types increased upon gamma-irradiation. In most cases a more or less pronounced decrease of extractable fatty acids can be observed after steam-sterilization of unsterilized and gamma-sterilized. This observation was strongest for extracts in DCM and ultrapure water. The use of ultrapure water seems to result in extraction of (most probably) surface-orientated fatty acids, rather than extraction out of the depth of the material.
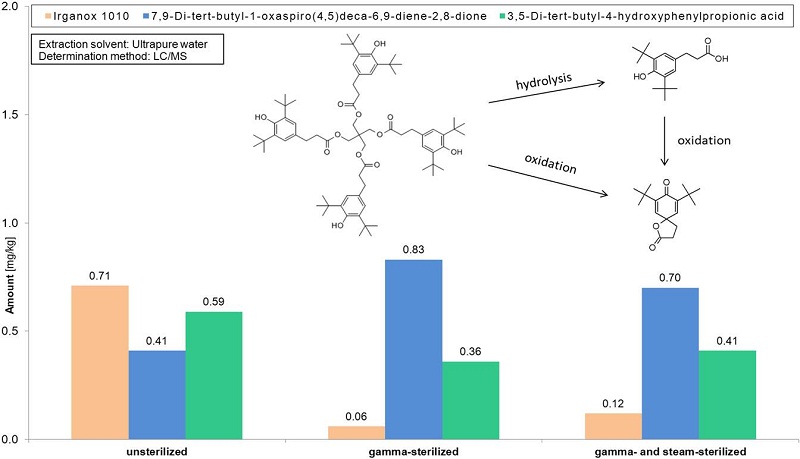
After gamma-sterilization of one sample, the concentration of water-extractable 7,9-di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione -one possibleIrganox 1010 degradation product – increases. This substance should be estimated as a potential Leachable, especially into aqueous drug formulations.A significant influence or trend of sterilization on the concentration of extractable cyclic rubber oligomers – e.g. C13H24 and C21H40 – was not observed for any examined sample.
Need for customized studies
The results of the study clearly demonstrate that sterilization of polymer components influences the respective extraction profile significantly. Thus, it can expected that the amount and the type of leachables from a primary packaging product that comes into contact with a pharmaceutical formulation also depends on the sterilization processing. Also, while this type of study is useful for giving trends, it is too general to fulfill the specific needs of an individual drug/container system. Therefore, customized E&L investigations should be executed on the basis of best practices extractables guidelines.
Laboratory service providers like SCHOTT pharma services can design and execute customized E&L studies based on best practice guidelines, and they also possess comprehensive know-how on the entire life cycle of commercially available primary packaging. This knowledge is crucial to determinethe origin of the substances found.
 Dr. Dan Haines
Dr. Dan Haines
Scientific Advisor for SCHOTT pharma services, earned his doctorate in Inorganic Chemistry at the University of Chicago. He joined SCHOTT in 2001 with a focus on developing glass coatings to control drug formulation interactions with glass surfaces. Since 2010 he is responsible for SCHOTT pharma services in North America providing analytical support of packaging material for pharmaceutical companies.
Dr. UweRothhaar
Director of SCHOTT pharma services earned his doctorate in Physics at the University of Kaiserslautern in Germany. He joined SCHOTT in 2000 and focused his activities on analytical support around glass and glass surfaces. Over the last years he is responsible for SCHOTT pharma services providing analytical support of packaging material for pharmaceutical companies.

Daniel Canton
Laboratory Manager for SCHOTT pharma services, earned his diploma degree in
Biotechnology with focus on Analytical Chemistry at the University of applied sciences in Darmstadt. He joined SCHOTT in 2011 as responsible person for development and conduct of Extractable & Leachable studies. During his diploma thesis he investigated extractable profiles of secondary packaging materials and their influence on the extractable profiles of primary pharmaceutical packaging materials. In 2015 he took over the function as manager of the Extractable & Leachable laboratory.





















