For many years, mass production has been the most prominent way to provide pharmaceuticals for all kinds of medical conditions. Large manufacturing sites have been established to produce vast amounts of uniform medications, with little room for customization. Alterations in the mass production line are often costly and lead to major downtimes – quite justly, since the focus lies on quantity at affordable costs.
Although pharmaceutical mass production still plays an indispensable role in the healthcare industry, novel advancements have led to targeted medicine emerging as a promising alternative in applications like cancer therapy or treatments of autoimmune diseases. The stunning potential of advanced therapies prompted researchers worldwide to focus on the development of products like antibody-drug conjugates, which have the potential to fight various types of cancer with outstanding effectiveness.[1]
Indeed, in 2000, the first ADC was approved by the FDA; by October 2023, 15 antibody-drug conjugates have received FDA approval, with numerous candidates currently being developed or tested. The laborious and intricate development process of ADCs, however, proves to be extremely challenging on lab scale, where there is still a high level of manual processing. Additionally, strict safety protocols are to be adhered to, protecting both staff and biologics from harm. In this context, automation in pharmaceutical settings emerges as a pivotal component. Innovative solutions for lab automation are put into place, with the aim of overcoming several barriers in ADC development related to formerly manual handling. This is about time, since the challenges related to ADC development are likely to increase along with their growing demand.[1] [2] [3]
Developing ADCs – requirements of different components
When developing ADCs, the three obvious components to deal with are antibodies, chemotherapeutic substances, and linkers to combine the former two elements. These main constituents of antibody-drug conjugates come with different requirements in terms of proper handling, with considerations to be made on safety and quality.
A main challenge in antibody-based products is temperature: Monoclonal antibodies like IgG – the most commonly used antibody type in ADCs – are prone to degradation if not stored adequately. Therefore, advanced cooling and freezing methods are to be implemented that are reliable and consistent. But especially when dealing with ultra-low sub-zero temperatures, proper training is necessary in order to protect both staff and antibodies.[5]
Maintaining sterility is necessary for all the components of ADCs – including the linkers that bind antibody and cytotoxic payload. These complex substances demand advanced laboratory techniques, as their quality is responsible for when and where the ADC releases its toxic payload upon administration. Thorough analysis, processing, and cleanroom conditions are therefore essential.[6]
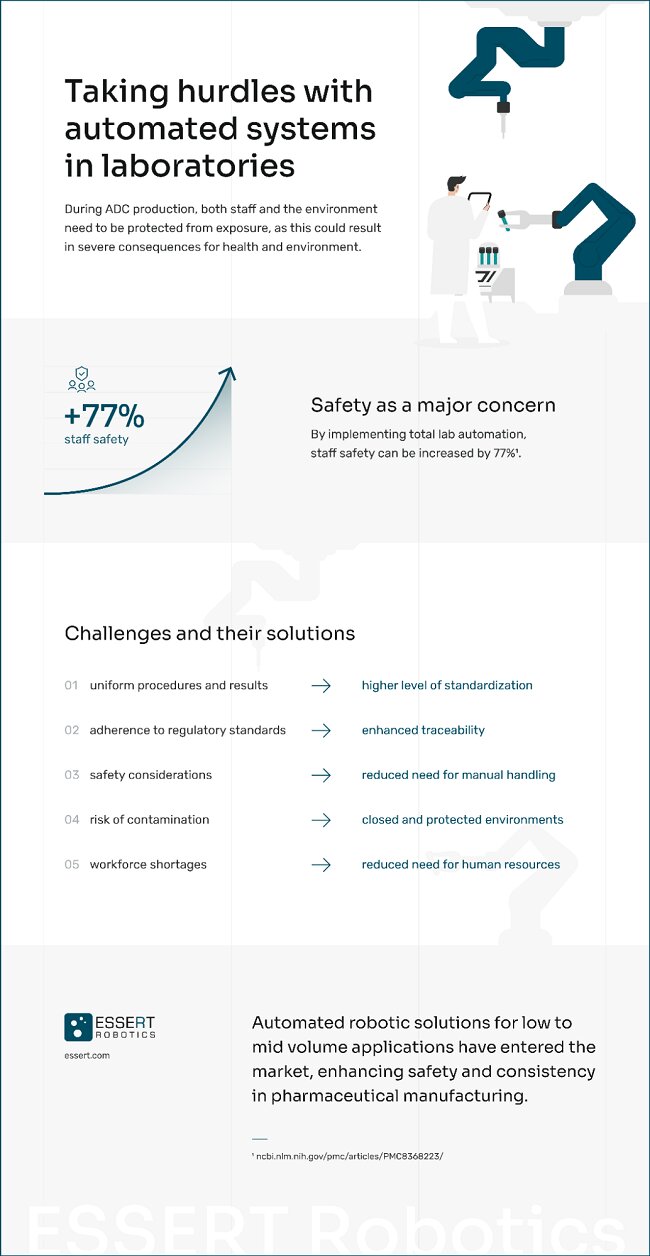
The probably highest-risk component of ADCs is the drug they carry: It is usually highly toxic, thus extremely effective when administered as a targeted therapy. During production, however, both staff and the environment need to be protected from exposure, as this could result in severe consequences for health and nature.
As a result, ADCs are not only a composition of different agents, but also a conglomerate of their individual requirements. Still, bringing these components together in the process of ADC production is not at all less challenging. And given the high levels of consistency and safety that need to be maintained, automated systems for bioprocessing need no longer be limited to large-scale production, but have to make their way to laboratories as well. Nevertheless, the uniformity, traceability, and safety they can bring to manufacturing processes are highly requested, reducing the risks related to manual handling.
Critical steps in ADC development
Although the exact execution of ADC development varies significantly, there are certain steps that are common to most processes in antibody production. This is where the expertise of laboratory staff comes into play: When developing an antibody-drug conjugate, all of these steps need to be optimized in the course of several production runs until they perfectly match – resulting in an ADC that is ready for application.
The intricate interplay of different production steps is a main topic in ADC research, illustrated, for instance, by a recent study with the purpose of designing an optimized ADC manufacturing process with anti-HER2 mAb drugs as an exemplary therapeutic.[7]
First, a cell line was chosen and prepared for monoclonal antibody production – an already complicated process in itself. Special cell culture conditions have to be maintained, and the expressed antibodies have to be purified and evaluated. Alternatively, many laboratories choose to rely on specialized suppliers, allowing labs to lay their focus on the ADC construction process.[8]
Once having established a suitable linker, the individual components of the ADC need to be combined, including the toxic payload chosen for a specific application – monomethyl auristatin E (MMAE) in the mentioned study. Yet again, this requires finding the ideal mixing conditions, including factors like temperature, time, and additional reagents.[9]
Automated systems may be used to precisely determine and adhere to predefined parameters, such as mixing time or different volumes of individual components. Only after further processing as well as being sure of satisfactory outcomes, ADCs can be prepared to leave the laboratory environment, being safely filled and packed. Even at this late stage, staff have to pay close attention to safety protocols and overall regulatory compliance. Thus, automated solutions that are designed for cleanroom operations are being introduced to achieve higher levels of safety and consistency.
Less is more – reducing manual labor as a game changer
Optimizing each of these steps in ADC development requires specialized knowledge, precision, and reproducibility. But bearing in mind the general hurdles that the biopharmaceutical industry has to take – an increasing demand for their products, while qualified staff is hard to find due to demographic changes – it becomes obvious that ADC development already and will increasingly challenge laboratories.
One viable way of taking these challenges is by reducing the amount of manual labor in laboratories. Innovative manufacturing solutions have entered the market that do not focus on creating a vast amount of uniform products, but rather automate specific parts in a lab-scale production chain while allowing high flexibility. Tasks like pipetting pharmaceutical liquids as well as mixing and dispensing them can be delegated to automated systems: Not only does this increase precision and traceability, but it also frees up human workforce for what they are really needed for. Additionally, staff safety in labs can be increased by 77% when switching to total lab automation.[10]
Medical device assembly, testing and packaging on lab-scale can be automated as well, ensuring compliance with regulatory standards with a minimum of resources. And by choosing modular solutions for automation, ADC development and manufacturing can be put on a future-proof footing.
References
1 Theocharopoulos C, Lialios PP, Samarkos M, Gogas H, Ziogas DC. Antibody-Drug Conjugates: Functional Principles and Applications in Oncology and Beyond. Vaccines (Basel). 2021;9(10):1111. Published 2021 Sep 29. doi:10.3390/vaccines9101111
2 Mythili Shastry et al., Rise of Antibody-Drug Conjugates: The Present and Future. Am Soc Clin Oncol Educ Book 43, e390094(2023). DOI:10.1200/EDBK_390094
3 October 17, 2023, ADC Approval up to 2023, Retrieved February 26, 2024, from https://broadpharm.com/blog/ADC-Approval-up-to-2023
4 October 17, 2023, Antibody Drug Conjugates (ADC) Market, Retrieved February 26, 2024, from htttps://www.marketsandmarkets.com/Market-Reports/antibody-drug-conjugates-market-122857391.html
5 Mythili Shastry et al., Rise of Antibody-Drug Conjugates: The Present and Future. Am Soc Clin Oncol Educ Book 43, e390094(2023). DOI:10.1200/EDBK_390094
6 Hotha, Antibody-drug conjugate development: challenges and opportunities, Retrieved February 26, 2024, from https://veranova.com/expert-insights/analyzing-antibody-drug-conjugates-with-kishore-hotha/
7 Ou J, Si Y, Goh K, et al. Bioprocess development of antibody-drug conjugate production for cancer treatment. PLoS One. 2018;13(10):e0206246. Published 2018 Oct 23. doi:10.1371/journal.pone.0206246
8 Ou J, Si Y, Goh K, et al. Bioprocess development of antibody-drug conjugate production for cancer treatment. PLoS One. 2018;13(10):e0206246. Published 2018 Oct 23. doi:10.1371/journal.pone.0206246
9 Ou J, Si Y, Goh K, et al. Bioprocess development of antibody-drug conjugate production for cancer treatment. PLoS One. 2018;13(10):e0206246. Published 2018 Oct 23. doi:10.1371/journal.pone.0206246
10 Kim K, Lee SG, Kim TH, Lee SG. Economic Evaluation of Total Laboratory Automation in the Clinical Laboratory of a Tertiary Care Hospital. Ann Lab Med. 2022;42(1):89-95. doi:10.3343/alm.2022.42.1.89






















