Antibody-drug conjugates (ADCs) are complex bioconjugates that combine a targeting antibody with a highly-potent payload using a linker molecule. This allows the delivery of nanomolar concentrations of a potent cytotoxic agent within a tumor (Figure 1). This targeted delivery to cancer cells limits toxicity to healthy tissues and increases the duration of activity of the therapy in the body, which limits side effects, bringing profound implications for cancer sufferers.
This drug modality has been revolutionizing cancer therapy, and the ADC market is expected to grow to $15.4 billion between 2024 and 2029[1]. To date, 14 ADCs have received market approval[2], and over 200 ADC-based candidates are in clinical development[3].
Due to their tripartite structure comprising a monoclonal antibody, cytotoxic payload, and a stable linker, developing and manufacturing ADC-based therapies can be challenging and costly. The manufacturing supply chain of these complex therapies needs to contain various technologies and manufacturing assets capable of supporting the microbial or mammalian expression of antibodies, chemical synthesis of highly-potent compounds, as well as a bioconjugation facility that can support the handling of cytotoxic compounds.
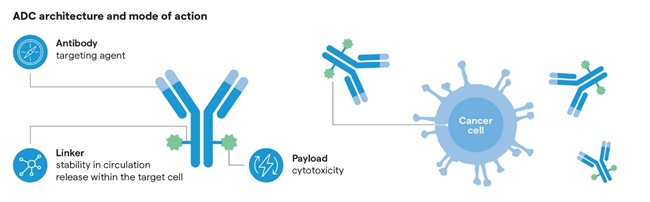
Overcoming bottlenecks of clinical and commercial manufacture of ADCs
The diversity in the design of ADCs directly translates to a diversity in technologies that are necessary for ensuring product quality, purity, sterility, and compliance with evolving regulatory requirements. In addition, considering the entire lifecycle of an ADC-based product that includes drug substance manufacture and drug product filling, an extremely low-temperature supply chain and secure labeling and packaging are necessary to mitigate product quality variations or issues arising from transport and storage-related damage[4].
Considering the number of processes involved, the manufacture of ADC-based therapies requires significant capital investments, decades of experience, and immense technical expertise across a multitude of platforms and technologies. In addition, establishing a reliable and high-performing logistics network for ADC production often requires years of effort.
As a result, drug developers often consider collaborating with experienced contract development and manufacturing organizations (CDMOs). Approximately 70% of drug developers focused on ADC development rely on CDMOs for progressing their molecules toward commercialization[5].
Identifying the right CDMO partner with specialized knowledge in the linker and payload technologies, as well as the necessary facilities for cytotoxic drug product filling, poses a significant challenge. Thus, choosing an industry partner with end-to-end ADC capabilities is essential to securing a consistent supply of materials from early clinical phases through to commercialization.
Recently, Lonza has heavily invested in all five technology areas needed for manufacturing bioconjugates, which led to an integrated ADC supply chain under one roof. The resulting offering spans drug substance and drug product manufacture for clinical and commercial supply, including an early development focus on payload and site-specific linker technology (Figure 2). To advance the DP capabilities, the offering was also expanded to include filling lines specifically designed to manufacture pre-clinical, clinical or small-scale commercial batches of highly-potent compounds. This approach aims to support the industry’s goal of advancing and commercializing these life-saving therapies to benefit patients worldwide.
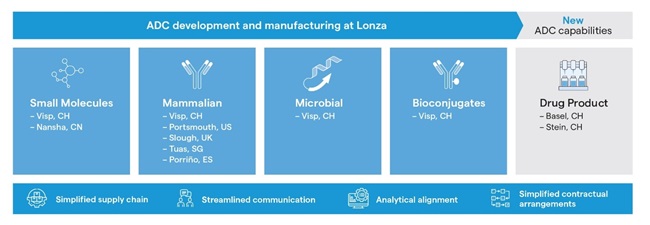
Accelerating ADC development
Advancing an ADC-based drug candidate towards commercial manufacture introduces unique hurdles, especially compared to the manufacturing setup of monoclonal antibodies (mAbs) or HPAPIs alone. Key challenges include managing toxicity, preventing contamination, controlling impurities, minimizing product loss, ensuring scalability, and maintaining regulatory compliance throughout the production process. These factors require stringent process controls and robust quality management systems at each stage, from intermediate synthesis to final drug formulation, to ensure consistent product quality and safety.
Simultaneously, while mAbs can generally be produced using established platform technologies, their biomanufacturing only represents one step within a complex, multistage ADC workflow, which often spans multiple CDMOs and locations. Effective coordination across these collaborations is crucial to ensuring the timely production of the final drug product for regulatory approval by health authorities.
If managed by multiple external partners, the journey from drug substance to drug product increases timelines for clinical trial applications, increases costs, and negatively impacts process design, mainly due to a lack of integration between these two manufacturing stages. Bringing the entire ADC lifecycle under one roof can significantly enhance the supply chain management and improve program timelines.
Full end-to-end integration streamlines overall chemical manufacturing control (CMC) management by enabling more efficient planning, program management, quality systems, analytics, storage, shipment, and logistics. This holistic approach enhances coordination across all stages, resulting in more seamless operations, improved timeline adherence, and reduced risk throughout the manufacturing process.
Lonza has been supporting ADC manufacturing since 2006. The recent acquisition of Synaffix[6], aimed at integrating an industry-leading technology platform and R&D capabilities combined with the integration of DP services for ADC products, further strengthens Lonza’s position at the forefront of innovation. Lonza’s integrated ADC offering[7] utilizes significant supply chain simplification and allows the delivery of DP in 15 months with an option to supply DS for toxicology studies in 8 months, including CMC data for IND filing[8]. This offering allows drug developers to progress according to their own risk profiles and milestones.
Speeding up ADC workflow
Significant time-saving during ADC manufacturing can be achieved by running multiple sets of workflows in parallel. At Lonza, the timeline of a given ADC program begins with the transfection of a DNA vector containing the mAb genes into an appropriate cell line for expression. Approximately two months after the cell line development, early mAb material is available for formulation development and small-scale bioconjugation studies. At approximately 3.5 months, the first material supply for pivotal conjugation development stages and formulation development is available.
A key milestone occurs at approximately seven months, when pilot-scale mAb material is produced, triggering the manufacturing of the ADC toxicology batch, which is also used as an interim reference standard for GMP release testing and analytical method validation.
The analytical method validation for the ADC runs in parallel with GMP mAb manufacturing. 12 months after the program begins, GMP drug substance (DS) can be released, with expected GMP DP material available after 15 months from the program start (Figure 3). Concurrently, regulatory documentation for the IND filing is prepared8.
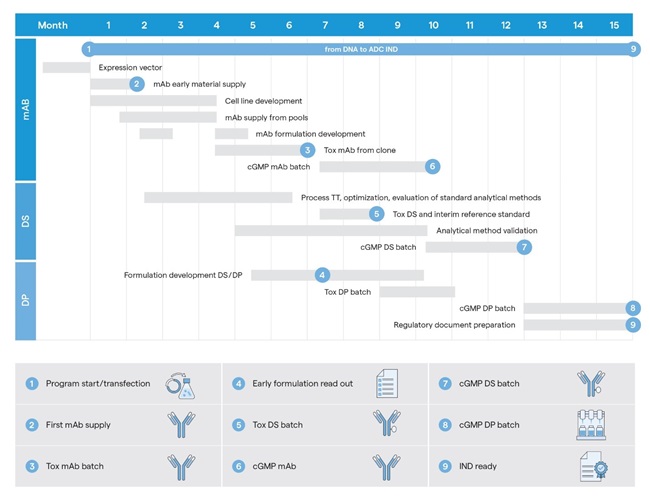
To expedite DS analytical method implementation and validation, our scientists leverage advanced platform-based analytical methods developed at Lonza, designed to address a wide range of analytical requirements within an ADC program. For DP development, a platform approach is applied to formulation studies, enabling early identification of the optimal formulation. This accelerates the execution of ADC toxicology batch production, where the formulation must already be fully defined and finalized. In addition, the optimized formulation mitigates risks associated with undesirable DP properties that could impact fill/finish processes, storage stability, or product administration.
ADCs continue to dominate the global bioconjugates pipeline by offering novel treatment options for patients with unmet medical needs, especially in the oncology field. The expected increase in demand for more targeted treatments in the autoimmune and oncology sectors will likely drive demand for novel ADCs. Due to their structure and a complex supply chain, these innovative therapies introduce challenges to development and manufacturing workflows. Drug developers lacking extensive in-house manufacturing capabilities, who are navigating the often fragmented landscape of ADC manufacturing can, therefore, greatly benefit from partnering with a CDMO that offers a comprehensive end-to-end service for ADC development and manufacturing. With careful planning and supply chain considerations, this holistic approach will help ensure the continuous supply of ADCs to meet patients’ needs.
References:
- Song, C.H., et al. Trends in the Development of Antibody-Drug Conjugates for Cancer Therapy. Antibodies (Basel). 2023;12(4):72.
- Fu, Z. et al. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Sig Transduct Target Ther. 2022; 7, (93)
- Clinicaltrials.gov Database Search for Clinical Trials with ADC, antibody drug conjugate | Recruiting, Not yet recruiting, Active,
- Ducry, L. Challenges in the Development and Manufacturing of Antibody–Drug Conjugates. Therapeutic Proteins 2012, 489-497.
- Antibody Drug Conjugate Market Size, Share & Trends Analysis Report by Application (Blood Cancer, Breast Cancer), By Technology (Cleavable Linker, Linkerless), By Region, And Segment Forecasts, 2021 – 2028. Published 2021.
- Lonza to Acquire Synaffix and Strengthen Antibody-Drug Conjugates Offering, published June 1st 2023. https://www.lonza.com/news/2023-06-01-07-00
- Ibex® Design ADC, DNA-to-IND Program, https://www.lonza.com/biologics/bioconjugates/ibex-design-adc
- For ADCs from DNA transfection to delivery of IND-enabling CMC modules. Subject to terms and conditions.
Author:
 Pierre Landais
Pierre Landais
Lonza Bioconjugates
Pierre Landais, Director of Commercial Development, Lonza Drug Product Services.
 Dr. Sandro Holzer
Dr. Sandro Holzer
Lonza Bioconjugates
Dr. Sandro Holzer, Head of Development, Lonza Bioconjugates




















