Medical device development is a long journey consisting of preliminary bench testing, multiple cycles of ‘build-test-fail-redesign’ and taking the product concept from Research and Development (R&D) to production. Other steps include analyzing the economic environment, getting through clinical trials, product validation, obtaining regulatory approval and more.
The costs associated with launching a medical device can be extreme, due to the sheer complexities in navigating regulatory and other market barriers.
Through the different stages of launching your medical device, an initial question is how important is the sterility Barrier System (SBS) in the grand scheme of things? Is it necessary to invest in adequate device protection, and how is this best achieved so the device arrives with sterility intact to the final customer?
The FDA sets rigorous standards and requirements to ensure the sterility of a medical device. With stringent standards and testing that require all packaging that is used with medical devices, medical device manufacturers must demonstrate the integrity of the sterile barrier throughout distribution to the point of use, keeping environmental factors and transportation dynamics that in mind.
When developing a medical device, engineers need to consider packaging as an integral part of the final product and understand that packaging plays a pivotal role in the successful launch of a medical device. Medical device packaging should be considered right from the start. A solid package design sets a strong foundation in the success of launching a new medical device to market.

Under Part 820, Quality System Regulation by FDA, section 820.30 (c), medical device design input is defined as follows:
‘Each manufacturer shall establish and maintain procedures to ensure that the design requirements relating to a device are appropriate and address the intended use of the device, including the needs of the user and patient. The procedures shall include a mechanism for addressing incomplete, ambiguous, or conflicting requirements. The design input requirements shall be documented and shall be reviewed and approved by a designated individual(s). The approval, including the date and signature of the individual(s) approving the requirements, shall be documented.’
In ISO 13485:2016, under section 7.3.3 Design and Development inputs, it highlights the need to establish the design and development inputs such as device description, usability requirements, substantial equivalence comparison, target product profile, labeling, and more.
Additionally, ISO 13485:2016, section 7.5.11, Preservation of Product says, ‘The organization shall protect product from alteration, contamination or damage when exposed to expected conditions and hazards during processing, storage, handling and distribution by: designing and constructing suitable packaging and shipping containers.’
With reference to device packaging, areas a packaging engineer would need to consider are the type of barrier required—single or double barrier—, the compatibility of the packaging material with the sterilization method, the stability of material after sterilization and aging, sale unit—single pack or multi pack— and distribution. All of the above requirements translate into material selection and technical specifications such as SBS dimensions, seal strength, and seal width—to name a few.
Type of Barrier – Single or Double
Whether a single or double barrier should be chosen depends greatly on how the device is transferred aseptically to the end user. An aseptic transfer of the device into the sterile field within an Operating Room (OR) / Emergency Room (ER) is of critical importance
in maintaining the sterile integrity of the device out of its SBS. An aseptic transfer can be achieved either by dropping the device onto the sterile field also called dumping, or via a scrub nurse presenting the device to the sterile nurse within the sterile field. In the case of requirements where the entire SBS containing the medical device needs to be transferred inside a sterile field, a double barrier (double SBS) becomes an obvious choice. In such cases, the outer barrier is removed just outside the sterile field and the inner
barrier containing the device is transferred into the sterile field by dropping or presenting, as explained earlier.
Sterilization Methods
One commonly used sterilization method in the healthcare industry is Ethylene Oxide sterilization (EtO), where medical devices are sterilized using gas. EtO is suitable for devices that have low resistance to water and heat, and when using EtO, some would prefer the SBS to have a porous side. The porous substrate allows the gas to penetrate the SBS surface, sterilize the device and exit out of the SBS by a de-gassing process. This process also ensures that there is minimal EtO residual on the device. Residual levels of the
gas are kept at a very low level to avoid any risk to the patient from the toxic and irritating effects of the gas. A SBS can come in a variety of designs such as pouches, rigid and semi-rigid trays. In such designs, typically one side of the SBS will consist of a porous material and the other side a non-porous material. The downside of this process is that it may take about 3-4 days for the sterilization cycle to be completed.
Another commonly used sterilization method is radiation. It is most commonly used on single-use devices such as syringes or catheters. This method is faster compared to EtO and more penetrating into the devices, so the downside to this type of sterilization is that the device may have some polymer degradation in terms of functional properties, usually exhibited in the form of discoloration. The most commonly used packaging materials that are compatible with EtO sterilization are equally compatible with sterilization via radiation as well. Orientation of the SBS during the sterilization process can be an important consideration for this method of sterilization.
Steam sterilization, though efficient, is often not the decontamination method of choice due to the possibility of the buildup of water droplets inside device components which can impair the functionality of the device or cause corrosion in materials that cannot come into contact with water. The choice of SBS material also differs from those typically used for EtO or radiation sterilization.
Some medical device manufacturers also select dry heat sterilization for devices such as in vitro diagnostics that are susceptible to water damage but resistant to heat. This method of sterilization requires higher temperatures but is very effective in neutralizing biological contaminants and spores.
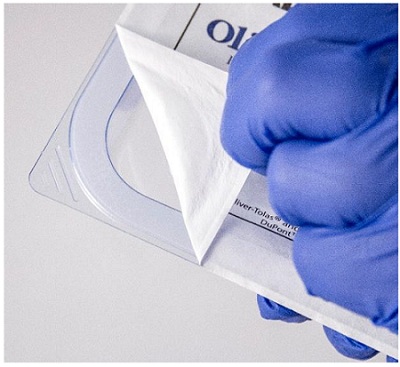
Seal Strength/Seal Integrity
Seals are a very critical part of the SBS because they prevent the entry of microorganisms and ensure the sterile integrity of an SBS from the point a final closure seal is applied until the point of use. As such, testing for seal strength is of prime importance. Integrity
tests check for pinholes or cracks on the SBS surface, as well as for channels and wrinkles across the width of the seal. Seal strength tests measure the mechanical strength of the seals in terms of force and ensure seal integrity is maintained during transportation. There are several American Standard Testing Method (ASTM) standards that describe methods to test SBS integrity and seal strength. ASTM D3078 describes a Submersion Leak method to test SBS surface integrity, whereas ASTM F1929 describes a Dye Penetration method to check for channels or wrinkles across SBS seals. For seal strength testing, ASTM F882 uses a Peel Test technique, and describes several methods to measure the force between two surfaces that form a seal.
There is no denying the fact that getting the right packaging and making sure it meets all ISO and/or ASTM standards is time consuming and complex. As a result, some medical device manufacturers have an entire team of packaging engineers who dedicate their time to identifying the appropriate packaging to ensure that medical devices remain in a sterile environment from distribution until the point of use.
At Oliver Healthcare Packaging, our customers rely on us to help them navigate the complexities of packaging and a reliable SBS. It is why our business is one hundred percent healthcare focused. Through innovation, and expertise in both technical support and customer care, we have partnered with thousands of medical device manufacturers to help them launch their medical devices successfully.
Speak to our technical engineers to get support on your packaging needs. From material recommendations to seal validation, we have the right expertise in place to help.





















