Positron emission tomography (PET) is a popular nuclear imaging technique used to gain information about specific molecular biomarkers. It is often combined with other imaging technologies, such as magnetic resonance imaging (MRI) and computed tomography (CT) scanning, to incorporate anatomical information. PET provides functional imaging, which shows the spatial distribution of biomolecular activity in living tissues and is therefore particularly useful for evaluating drug candidates in preclinical studies.
PET creates three dimensional (3D) images of the subject using radioactive tracers – molecules bound to a radioactive isotope – that are usually injected intravenously (iv). The carrier molecule can interact with or bind to specific proteins, receptors, and biomolecular pathways in the body, to quantify a specific biological activity. The radioactive isotope, commonly fluorine-18 (18F) or carbon-11 (11C), produces positrons that interact with the surrounding electrons, resulting in the annihilation of both particles and the release of two photons (gamma rays). These photons emit in opposite directions (~180°) and are picked up by detectors in the PET scanner to map the radionuclide distribution in the body.
In preclinical pharmaceutical research, the non-invasive, sensitive and quantitative nature of PET imaging is utilized to advance knowledge of diseases and drug activity in the body. Successful drug development relies on the ability to understand dynamic biological processes, gene expression, enzyme and protein activity, progression and treatment of diseases, biodistribution, and pharmacokinetics/pharmacodynamics of new drugs. The multi-modal imaging approach of PET/CT, provides a method to map the path of drugs throughout the body over time, to monitor efficacy and establish suitability for clinical use.
Preclinical oncology research
Evaluating therapeutic efficacy of drug candidates
PET/CT has been used in preclinical oncology investigations into the efficacy of potential novel treatments. For example, one study used small animal PET/CT imaging to confirm the preclinical efficacy of fasudil – an approved drug for cerebrovascular bleeding – for inhibiting tumor growth in gastric cancer (GC)1. Female transgenic mice were injected with 18F-fludeoxyglucose (18F-FDG), a glucose analogue tracer, four times a week for four weeks. The mice were deprived of food for 3 hours and then anesthetized and iv injected with 18F-FDG for PET/CT imaging. For optimal tracer distribution the mice were kept under anesthesia for 60 min below a red warming lamp and imaged using small animal PET/CT/SPECT system (Bruker’s Albira II). The overlay of the PET/CT images from treated and control mice (Figure 1) demonstrated that the gastric tumor was localized below the heart and between the kidneys. Quantitative calculation of the 3D tumor volumes showed a reduction in 18F-FDGuptake and signal intensity by fasudil, compared to non-treated control animals (Figure 1). The results of the study show that fasudil is a viable novel strategy for the treatment of GC.
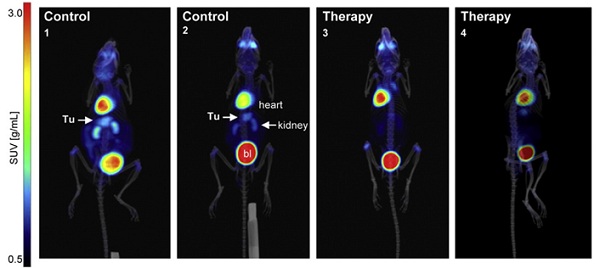
Figure 1: Preclinical efficacy of fasudil in gastric tumors of transgenic mice with gastric carcinoma (CEA424-SV40 Tag). 3D PET/CT in situ detection of gastric tumors in mouse stomachs. Signals from 18F-FDG uptake in fasudil-treated (4 weeks) and control mice presented as graded color code with standard uptake value (SUVbw) range of 0.5-10. (Tu = tumor, bl = bladder. Arrows show the gastric tumor between the two kidneys and below the heart. Reproduced from reference [1] under the Creative Commons License 4.0 https://creativecommons.org/licenses/by-nc-nd/4.0/
ImmunoPET and personalized medicine
Another preclinical oncology study looked at imaging receptor expression in cancer models with microPET/CT, specifically for the treatment of triple-negative breast cancer (TNBC)2. The heterogeneity of TNBC and lack of actionable targets make treatment challenging, and there is therefore an unmet clinical need for new molecular targets for TNBC. The literature suggests that AXL – a member of the receptor tyrosine kinase TAM subfamily – plays a role in TNBC and other cancers, and could be investigated as a potential therapeutic target.
Using microPET/CT (AlbiramicroPET/SPECT/CT, Bruker BioSpin), expression of AXL and its downregulation by 17-allylamino-17-demethoxygeldanamycin (17-AAG) – a potent inhibitor of heat shock protein 90 (HSP90) – could be imaged and quantified using copper-64-labelled anti-human AXL antibody (64Cu-anti-hAXL) as a radioactive probe, shedding light on therapeutic efficacy for AXL-targeted molecular therapies (Figure 2). Both PET imaging and radionuclide therapy are made possible by the short half-life (~13h) and β+ and β− emissions of 64Cu, making it a desirable PET radionuclide for antibody and nanoparticle labeling.
The in vivo imaging experiment showed that 64Cu-anti-hAXL had greater tumor uptake and accumulation than non-specific 64Cu-IgG, suggesting that 64Cu-anti-hAXL specifically binds to AXL-expressing tumor cells. By using 64Cu-anti-hAXL as an imaging probe, the researchers demonstrated that non-invasive assessment of AXL expression using microPET/CT in TNBC may be used to predict drug resistance and response to therapies directed at AXL. Such results may be used to develop theranostic agents in the future, which enable a specific diagnostic test before delivering therapy to a particular molecular target on the tumor.
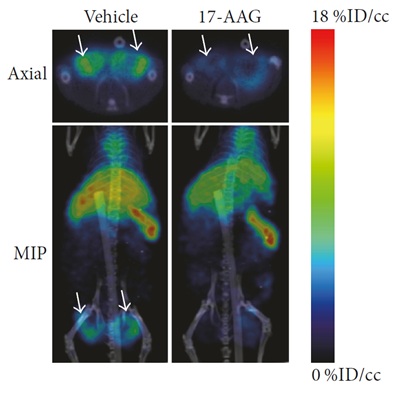 Figure 2: MicroPET/CT images of MDA-MB-231 tumor xenografts in mice 24 hours after intravenous injection with 64Cu-anti-hAXL. Treatment group received daily 17-AAG injections and showed lower tumor uptake of 64Cu-anti-hAXL than the vehicle-treated control group. Reproduced from reference [2] in accordance with Creative Commons License 4.0 https://creativecommons.org/licenses/by/4.0/
Figure 2: MicroPET/CT images of MDA-MB-231 tumor xenografts in mice 24 hours after intravenous injection with 64Cu-anti-hAXL. Treatment group received daily 17-AAG injections and showed lower tumor uptake of 64Cu-anti-hAXL than the vehicle-treated control group. Reproduced from reference [2] in accordance with Creative Commons License 4.0 https://creativecommons.org/licenses/by/4.0/
The combination of PET and CT imaging is a valuable tool in oncology research due to its ease-of-use, high-throughput capabilities and high resolution for bone and pulmonary applications. These studies place PET/CT at the forefront of cancer therapeutics development and tumor biology research, for cancers with high mortality rates such as GC and TNBC.
Future developments
Developments in PET, MRI and CT imaging, as well as ongoing research into new radiopharmaceuticals for in vivo imaging, have enabled the non-invasive evaluation of new potential therapies, for a broad range of diseases. The studies presented in this paper highlight the potential for PET/CT imaging to determine the mechanism of action of a drug and monitor biomarkers to assess a drug’s therapeutic efficacy.
The physiological, pharmacological and biochemical measurements that are made possible by PET/CT imaging are propelling drug development in preclinical studies. Harnessing the power of nuclear molecular imaging with advanced instrumentation allows pharmaceutical researchers to progress potential drugs through preclinical studies and secure the development of tomorrow’s therapies.
For more information on Bruker’s preclinical imaging solutions, please visit https://www.bruker.com/products/preclinical-imaging/nuclear-molecular-imaging.html
References
- Hinsenkamp I, Schulz S, Roscher M, Suhr A, Meyer B, Munteanu B, Fuchser J, Schoenberg SO, Ebert MPA, Wängler B, Hopf C and Burgermeister E (2016) Inhibition of Rho-Associated Kinase 1/2 Attenuates Tumor Gorwth in Murine Gastric Cancer, Neoplasia, 18;500-511.
- Wang W, Zhao J, Wen X, Lin CC, Li J, Huang Q, Yu Y, Lin S and Li C (2017) MicroPET/CT Imaging of AXL Downregulation by HSP90 Inhibition in Triple-Negative Breast Cancer, Contrast Media & Molecular Imaging, vol. 2017, Article ID 1686525, 11 pages.

















![Sirio Launches Global Research Institute for Longevity Studies [SIA]](https://www.worldpharmatoday.com/wp-content/uploads/2019/09/Sirio-218x150.jpg)



