Ultra high barrier films protect any dosage content
The Pharmaceutical Industry has always mainly focused on novel drug research, formulation development, and subsequent clinical trials. As with all other phases it presents its own challenges. There is no use if packaging does not maintain the drug activity until it reaches the patient. Currently the role of packaging had expanded beyond protection and maintenance of product integrity. It has to also comply with major regulatory authorities such as Code of Federal Regulations ( CFR ) , us Guidance for Industry , Container Closure Systems, and EMA Guidance : Plastic immediate packaging materials.In the last two decades there is an increasing demand for packaging solutions that improve product shelf life while maintaining product integrity during distribution.
The emerging trend of Ultra-High–Barrier Films
The demand for highly protective packaging solutions has fueled the development and manufacturing of Ultra- High- Barrier packaging films. While meeting all regulatory needs with although higher costs they off-set in the long term reduction in hidden costs. There are currently multiple options of ultra-High – Barrier films and pharmaceutical packaging engineers often find it difficult to understand the specific application of each of these products. The packaging requirement of any pharmaceutical drug is mainly governed by the nature of the drug product and its type of formulation, target market and data available from the innovator.The present article presents to guide pharmaceutical packaging engineers in deciding when to use ultra- high- barrier-films , the best practices in working with Ultra- High- Barrier – Films to ensure a smooth packaging operation , and last but not the least , different methods for testing packaging integrity.
Mono – Aclar – A key player in Ultra- High – Barrier – Films
Mono- Aclar films manufactured by Honeywell, have been well know and trusted for over 40 years by the world’s largest pharmaceutical companies. Primarily Aclar is a polychlorotrifluoroethylene (PCTFE) flouropolymer. They are optically clear, biochemically inert, flex –crack and chemical resistant, un-polar, non- flammable, free of plasticizers and stabilizers, with good forming and non- altering properties. Table 1 compares different properties of Aclar and those of PVC.
The transformation of Mono- Aclar to ACG Aclar Laminates
ACG has more than two decades of experience in pharmaceutical packaging. ACG has been catering to diverse industries in more than 80 countries, including, pharmaceutical, health care, personal care and medical device sectors. By lamenting mono-Aclar with different appropriate films, ACG has introduced various ultra high barriers, named ACG Aclar laminates, which meet specific barrier requirements of each formulation and offer the highest protection against environmental factors.
Since ACG Aclar laminates are inert, they offer the best options for packaging of highly reactive drug formulations. Another advantage of ACG Aclar laminates is that its push-through force is equivalent to other ultra –high –barrier films approved by the US and European regulatory authorities
Types of Laminates
- PVC/ ACLAR
- PVC/PE/ ACLAR
- PVC/EVOH/ACLAR
- APET/ACLAR
- APET/ EVOH/ ACLAR
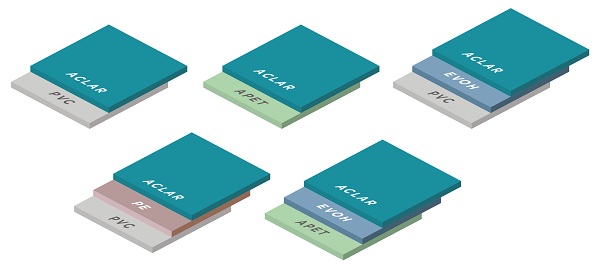
Depicts the ACG Aclar laminates most commonly used in packaging
Working with ACG Aclar Laminates
The barrier properties of ACG Aclar Laminates can alter during the actual thermoforming process. The recommendation below will give you the best results when working with ACG Aclar laminates.
• Thermoforming of ACG Aclar Laminates
Typical forming temperatures for ACG Aclar Laminates ranges from 110C to 140C and sealing temperature ranging from 160C to 190C. These forming and sealing temperatures depends on speed of machine, available change parts, size of cavity and film structure being used.
• Use a flat forming machine instead of a Rotary one
A high pressure ( 6-8 Bar ) is needed for the formation of ACG Aclar laminates since they are one of the most durable high barrier films with a high melt flow index.
• Use Plug Assists
The thermoforming process of ACG Aclar is further simplified with plug assists, which allow proper cavity formation with appropriate thickness.
• Use a dedicated mold instead of a universal mold.
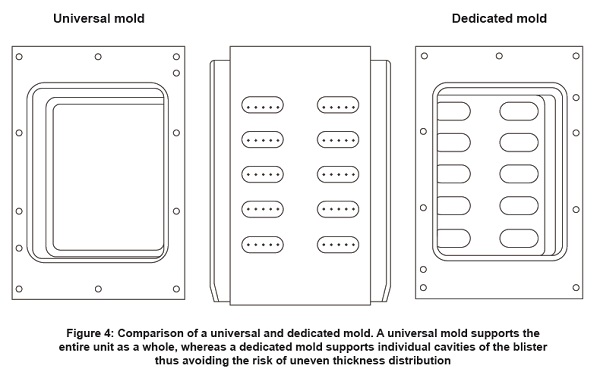
When thermoforming change parts are taken into consideration, dedicated molds are highly recommended over universal ones. Figure 4 because of an array of advantages they provide.
- Uniform thickness distribution
- Reduced stress formation
- Ensures controlled Forming
- Consistent Flange Width
- Avoid Non- Homogeneous cooling
- Minimize risk of micro- channeling in the sealed area
Packaging Integrity Tests of ACG Aclar Laminates
For blsters form, it is imperative to check whether the packaging offers the required protection, and this can be determined only by packaging integrity tests.
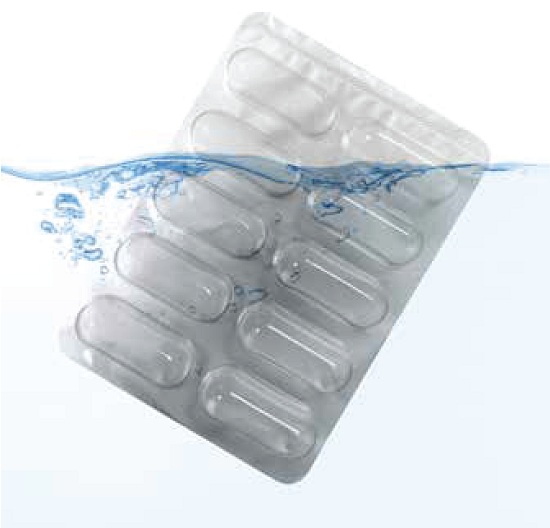
• Leak Detection Test
Also referred to as ‘ Methylene Blue Test ‘ , this method uses a vacuum chamber partially filled with a mixture of water and Methylene blue dye. The blisters are submerged in this liquid and held steady for a specific period at a specific level of vacuum drawn on the chamber. After the chamber is vented to the atmospheric pressure, cavities /seal areas of the blisters are examined for any sign of the blue dye.The major drawback of this test is that it is a destructive test because the samples tested cannot be used for weight gain test , and therefore requires statistical sampling to ensure that an acceptable number of blisters are detected.
• High Intensity Light Test
This simple test examines cracks in the foil. It is conducted in a dark room where a flash light is shinned through one side of the sealed blister. If there is no light coming through the foil and plastic, it means there is no pin holes in the seal.
• Weight Gain Test
This is by far the most important test for a blister pack even though it is time consuming, taking approximately 40 days. It measures the moisture permeability of packages filled with desiccant irrespective of the dosage amount. The blisters are kept in a labeled package holder individually, and their initial weight is measured (day Zero). Then the blisters are placed in a humidity chamber as per ICH conditions. Samples are weighed everyday for the next ten days at least every other day. After 10 days reading frequency can be reduced to once a week.The results are reported as weight gain (WGdayX ) in g package and plotted on a graph. A linear data indicates favorable results whereas Non-linearity may indicate a problem with the samples, saturated desiccant tablets, or the data collection method.
Conclusion
Ultra-barrier-Films are gaining popularity in the pharmaceutical industry owing to their ability to provide ultimate protection to pharmaceuticals against environmental factors, thereby boosting their shelf life. Although different types of ultra-high- barrier films are available in the market, packaging engineers still face the problem of selecting the right kind for the intended product because of the pressing timelines. To select the right barrier film, packaging engineers should first isolate the impurities that form in the products and identify the underlying reasons, such as moisture, oxygen, and light. The type of barrier film then can be selected accordingly.ACG Aclar laminates is one of the well known ultra- high- barrier packaging materials and is available in various types to meet every barrier requirement of pharmaceutical products. By following the best practices , ACG Aclar laminates provides the optimum barrier performance.

















![Sirio Launches Global Research Institute for Longevity Studies [SIA]](https://www.worldpharmatoday.com/wp-content/uploads/2019/09/Sirio-218x150.jpg)


