Patient recruitment is often cited as one of the biggest challenges faced by clinical trial professionals. Many clinical trials fail because sponsors cannot recruit sufficient patients to reach their target sample size[1,2].
In fact, only 55% of trials reach enrolment targets within their specified patient recruitment period [1]. This causes extensions and time delays which can cost biopharmaceutical companies up to $8 million a day in lost sales [3]. The problem of patient recruitment is industry-wide, affecting all disease areas and all countries in which clinical trials take place[2, 4]. It is further complicated by patient dropout rates of up to 40% in longitudinal studies.
Biopharmaceutical companies spend considerable time and money on patient recruitment and retention.The average cost to recruit and retain patients for clinical trials of a single drug is nearly $900,000 [2]. Despite this investment, many patient recruitment efforts fail.
Why is Patient Recruitment Such a Significant Problem?
There is a large body of scientific literature examining the challenges of patient recruitment and discussing potential solutions to this industry-wide problem. Many factors contribute to difficulties in recruiting and retaining patients. The biopharmaceutical industry is becoming increasingly competitive, and often two or more large companies run simultaneous clinical trials in the same disease area.Consequently, these companies must compete for the same population of patients [2]. Patients and physicians are not always aware of, or understand the benefits of new clinical trials [2, 4]. Clinical trial failures are often widely publicized in the mainstream media which can cause mistrust in the general population.Certain community groups may have social and cultural aversions to clinical trials [2, 4].
Many solutions have been proposed to solve the challenges of patient recruitment and retention. These include: increasing public awareness through videos, podcasts, advertising and social media; offering incentives to clinical trial staff and participants; training staff on patient engagement; face-to-face discussions with patients; and trialing new patient engagement methods such as gamification, medical animations and patient engagement platforms[5]. However, due to the complex nature of clinical trial design, most patient recruitment studies have been qualitative and few proposed solutions have been rigorously tested by quantitative, controlled scientific experiments [5].

Sample Repurposing Can Solve the Problem of Patient Recruitment
Reusing stored clinical samples in multiple non-interventionalresearch studies can relieve some of the burden of patient recruitment.In the last decade, research technologies and tools have advanced, and many newer assays are significantly more sensitive, and require less starting material than older technologies. Therefore, it is now possible to generate more data fromclinical samples. With the correct patient consent, samples can be aliquoted and used across multiple studies. Many government and medical research funding agencies advocate for the reuse of clinical samples. The Medical Research Council in the UK recently published ethical guidelines stating that research organizations should actively maximize the research use of all research samples [6].
Sample repurposing offers significant financial and time-saving benefits to biopharmaceutical companies. It reduces not only patient recruitment and retention costs, but also physician and clinical staff costs, clinical procedure costs and clinical trial site costs. Together, these factors can add up to more than 50% of the total cost of a clinical trial [2]. Therefore, sample repurposing can translate into hundreds of millions of dollars in savings. Reusing existing samples can also accelerate clinical trial timelines as it can take years to organize facilities and recruit staff and patients for a new clinical trial [2].
Careful planning is required to reuse clinical samples. Under current US law, de-identified samples can be used in new research studies without further consent [7]. However, this may change in the future. Furthermore, other regulatory regions require adequate informed consent from patients before their samples can be repurposed. This is called‘ broad and enduring’ or ‘generic’ consent[6]. Broad consent is not considered valid in some EU member states [8]. Therefore, if conducting trials in those countries, sponsors could use two-part consent,which asks for separate consent for the planned study and for storage and future use [6]. Informed consent protocols shouldbe culturally sensitive to all patients[9].
Sample processing and storage procedures will also affect whether clinical samples can be used in future studies[6].Sample integrity impacts the quality of any data generated from that sample[10]. For clinical samples to be successfully repurposed, they must be collected, stored and transported using procedures that maintain sample integrity and comply with international regulatory requirements.
Sample Hub™Integrated Lifecycle Management
Integrated, standardized clinical trial sample management allows companies to repurpose samples for multiple research studies. Housing samples within a single multicenter platform can streamline workflow, accelerate clinical trial timelines and maximize the value of clinical samples.
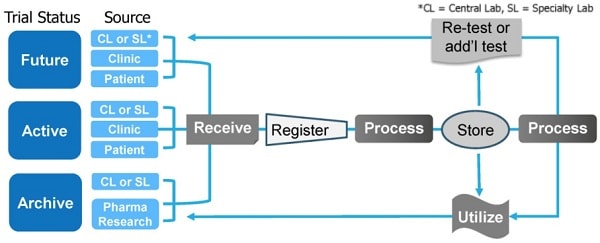
Caption: Brooks Life Sciences’ Sample Hub platform offers integrated clinical sample management solutions to help solve the problem of patient recruitment and reduce the cost of clinical trials.
Brooks Life Sciences’ Sample Hub platform is a global interconnected network of sample storage, transport and processingbiorepositories. The biorepositories are staffed by a team of experts whocan help clients navigate the complex international regulatory landscape when designing and running multicenter clinical trials.Sample Hub facilities and staff operate with standardized training, procedures, protocolsand quality control systems. This ensures all sample handling and data generation is standardized across multicenter clinical trials and across multiple clinical trials.
Sample Hub is a flexible, integrated platform that can be customized to each client’s individual sample management needs. This includes managing sample handling, analysis and storage for active studies; archiving samples for completed studies; and planning and project managing future studies. By consolidating samples within the Sample Hub platform, biopharmaceutical companies can reduce the cost and timeline of clinical trials.
This is because samples are stored, transported and analyzed within a single platform which streamlines workflows and minimizes sample handling delays. Samples can be retrieved within 24 hours and all vendor relationships are managed at a central point, sending samples to central labs, specialty labs or CROs as required. This integrated system ensures sample integrity, chain of condition, chain of identity and chain of custody are maintained throughout the sample management process. The platform also offers connected informatics and real-time sample tracking.
By repurposing clinical samples and using an integrated, standardized approach to sample management, such as the Sample Hub platform, biopharmaceutical companies can help reduce the problems of patient recruitment and lower the cost of clinical trials.
References
1. Sully, B.G., S.A. Julious, and J. Nicholl, A reinvestigation of recruitment to randomised, controlled, multicenter trials: a review of trials funded by two UK funding agencies. Trials, 2013. 14: p. 166.
2. Sertkaya, A., et al., Examination of Clinical Trial Costs and Barriers for Drug Development. 2014, U.S. Department of Health and Human Services.
3. The Expanding Web of Clinical Trial Patient Recruitment. 2014.
4. Kadam, R.A., et al., Challenges in recruitment and retention of clinical trial subjects. Perspect Clin Res, 2016. 7(3): p. 137-43.
5. Bower, P., et al., Interventions to improve recruitment and retention in clinical trials: a survey and workshop to assess current practice and future priorities. Trials, 2014. 15: p. 399.
6. Human Tissue and Biological Samples for Use in Research: Operational and Ethical Guidelines, M.R. Council, Editor. 2014, Medical Research Council.
7. Federal Policy for the Protection of Human Subjects, U.S.H.a.H.S. Department, Editor. 2018.
8. Sariyar, M., et al., Sharing and Reuse of Sensitive Data and Samples: Supporting Researchers in Identifying Ethical and Legal Requirements. Biopreserv Biobank, 2015. 13(4): p. 263-70.
9. Tindana, P., et al., Ethical issues in the export, storage and reuse of human biological samples in biomedical research: perspectives of key stakeholders in Ghana and Kenya. BMC Med Ethics, 2014. 15: p. 76.
10. NCI Best Practices for Biospecimen Resources. 2016.






















